A preliminary report from the 100’000 Genomes pilot suggests that the use of Whole-Genome Sequencing (WGS) could benefit healthcare. The report concludes that through WGS, the diagnostic yield across a range of rare diseases could be increased. Thus deliver patients with rare illnesses with a diagnosis, provide reassurance for parents that they did not pass their conditions on to their children, or in some cases, lead to life-changing treatments [1,2].
Rare diseases, despite the name, impact more lives than we tend to think. Almost 1 in 20 individuals suffer from a rare disease, with many undiagnosed cases and no cure available for plenty of these conditions.
Whole-Genome Sequencing (WGS) is a sequencing technique for analyzing the entire genome. It is based on the massively parallel sequencing technology, mostly known as Next-generation Sequencing (NGS), that in the last decades has revolutionized genomic research. NGS provides high-throughput and scalable methods supporting a broad set of applications in research and diagnostic.
The study, funded by the National Institute of Health (NHS), included the sequencing of 4’660 genomes provided from the 2013 launched 100’000 Genomes Project by the British government. Targeting rare genetic variants to find explanations for rare diseases, this study is one of the first to investigate the impact of WGS in healthcare systems [1].
The results led to an increase in the diagnostic yield to more than 40%, especially for patients with vision and hearing disorders or conditions for intellectual disability. Overall, a quarter of participants, many of whom endured a long odyssey of medical assessments without an explanation for their situation, now received a diagnosis resulting in more focused care through adjusted treatments or therapies [2].
In parallel with the direct positive effects for patients, WGS can also help relieve the healthcare system in terms of costs, as a WGS-based approach can speed up diagnostic time and thus gain valuable time for treatment and reduce lengthy hospital stays. In one example cited in a Guardian article, the estimated costs for a patient’s hospital visits and intensive care admissions reached £ 356’571. In contrast, a whole-genome analysis identifying the underlying cause and treatment cost £ 70’000 [2].
Although 25% of patients finally received an answer on their condition, 75% are still in the dark about the cause for their situation. But there is still hope coming from the genomic research progress, continuously providing insight about the genetic basis of human diseases. For example, today, ~84 annotated and thus more explained variants are published each month in a single database. Therefore, availing of their genome sequencing data could help deliver a diagnosis in the near future to a growing number of patients [2]. At GenomSys, we have been observing this development for some time and we would like to do our part in this regard. With several initiatives around the world – similar to the 100,000 Genomes Project – there are additional new challenges, such as handling the vast amount of data.
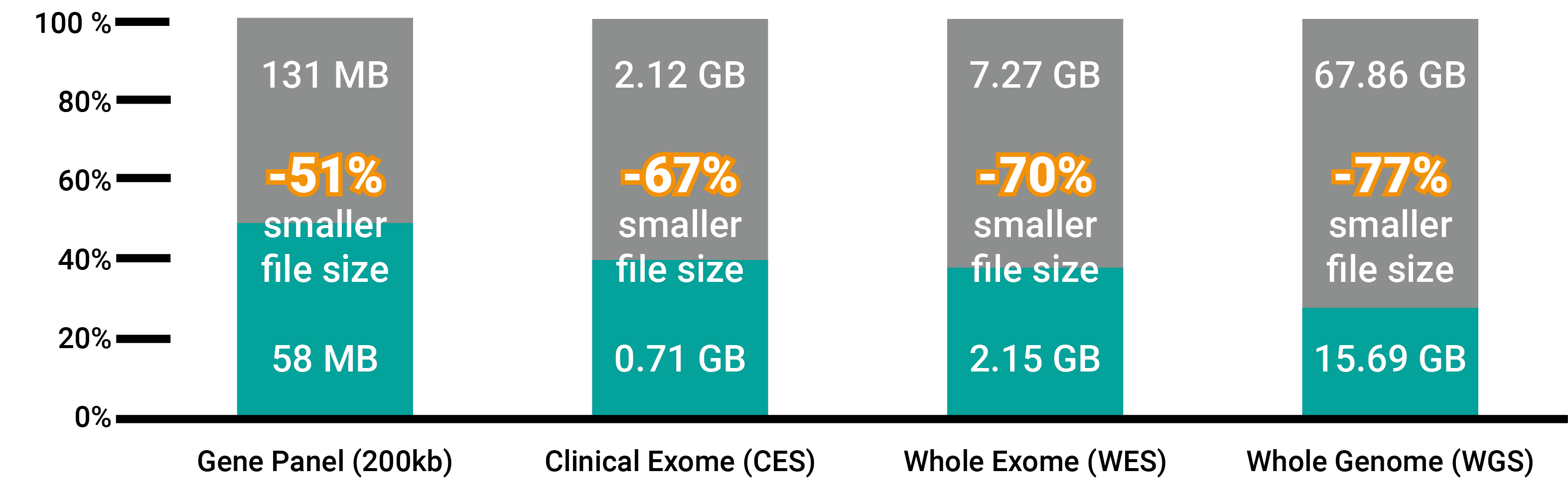
Today a whole-genome dataset makes up to 67 GB of storage space in a legacy genomic data format. For the 100’000 Genomes Project alone, this would mean 6’700’000 GB to store the datasets. An incredible amount of energy and storage capacity would be needed. Therefore, amongst a group of international companies and institutes, GenomSys developed together with the ISO committee a standard for the representation of genomic data (MPEG-G). One of the standard’s benefits is the compression of data. The same dataset would be compressed to less than 16GB, meaning a reduction of 77%.
 As the possible impact of WGS become more and more investigated and relevant in the clinical setting, the advantages concerning the economic side of healthcare are an essential part of how the future of medicine and care will look. Apart from the faster turnaround times in delivering (finally) a diagnosis to the patient and thus saving precious time, the approach for handling the genomic data-efficient should be the goal from day one.
As the possible impact of WGS become more and more investigated and relevant in the clinical setting, the advantages concerning the economic side of healthcare are an essential part of how the future of medicine and care will look. Apart from the faster turnaround times in delivering (finally) a diagnosis to the patient and thus saving precious time, the approach for handling the genomic data-efficient should be the goal from day one.
One of our solutions, GenomSys Codec Suite, a tool to transform legacy genomic data formats into the more efficient and future-proof MPEG-G format, reflects that.
We foresee a rapid increase in data in the future – as we had the glimpse already in the COVID-19 pandemic – particularly in genomics. Technology handling the data will be indispensable. By implication, this means that if these tremendous new methods like Whole-genome Sequencing can support professionals in their quest to improve patients’ lives, we want to provide them with the foundation on which the technology can run more efficiently and sustainable in energy uptake.
By Lucas Laner on December 14, 2021.
References:
[1 Drs. Smedley and Smith, Mr. Martin, and Drs. E.A. Thomas, McDonagh, Cipriani, Ellingford, Arno, Tucci, Vandrovcova, Chan, and H.J. Williams and Drs. Scott, Fowler, Rendon, and Caulfield. 100,000 Genomes Pilot on Rare-Disease Diagnosis in Health Care — Preliminary Report. N Engl J Med 2021;385:1868-80. DOI: 10.1056/NEJMoa2035790[2] Linda Geddes. Whole genome sequencing could save NHS millions of pounds, study suggests (2021). https://www.theguardian.com/science/2021/nov/10/whole-genome-sequencing-could-save-nhs-millions-of-pounds-study-suggests
Picture: MasterTux/ pixabay





